The Rise of Hemp Litigation: Courts Hit Pause on CBD Lawsuits
Reviewed in this article: Pittman et. al. The Rise of Hemp Litigation and the Primary Jurisdiction Doctrine. The Judges' Journal; Chicago Vol. 60, Iss. 1, (Winter 2021): 37-40. ($34.95)
Since the 2018 Farm Bill legalized hemp-derived products, the marketplace has been flooded with CBD oils, gummies, tinctures, and beverages. Alongside this boom came a wave of lawsuits. Everything from consumers alleging mislabeling, to false advertising, or unapproved health claims. Yet, in courtrooms across the country, many of these cases have been put on hold. Why? Enter the primary jurisdiction doctrine.
What is the Primary Jurisdiction Doctrine?
The primary jurisdiction doctrine allows a court to pause a case when a government agency—here, the Food and Drug Administration (FDA)—has the expertise and authority to decide the central issues. In CBD cases, judges often decide that before they can rule on whether a label is misleading or whether CBD can be sold as a supplement, the FDA must first issue clear rules.
Real Cases, Real Outcomes
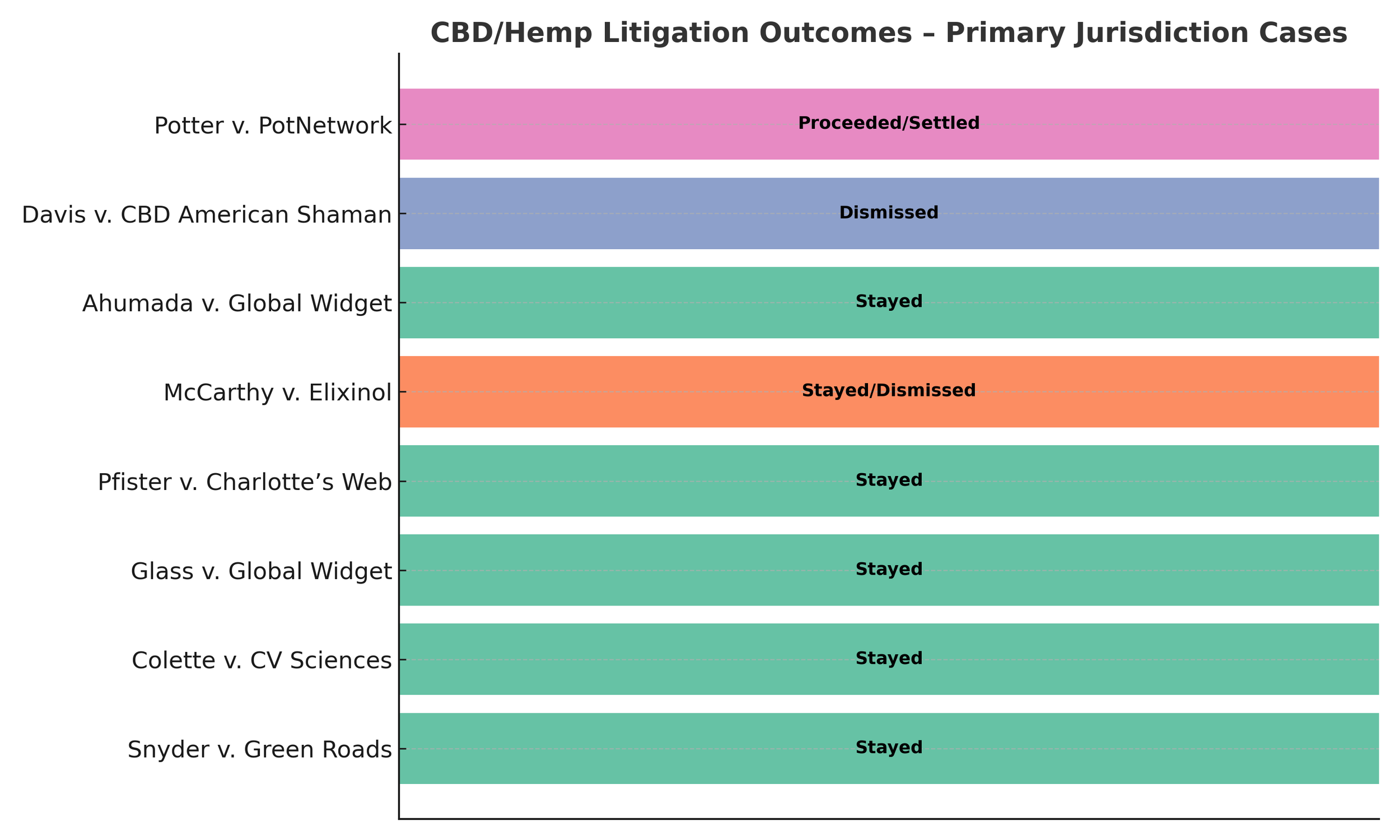
Courts from California to Florida have applied this doctrine in CBD lawsuits:
Snyder v. Green Roads (2020, Florida): Case stayed because FDA had not yet defined whether CBD could be legally sold in foods or supplements.
Colette v. CV Sciences (2020, California): Stay issued to avoid inconsistent rulings across the dozens of similar CBD class actions.
Pfister v. Charlotte’s Web (2020, Illinois): Litigation paused until FDA clarified what “hemp extract” meant for consumer expectations of potency.
Ahumada v. Global Widget (2020, Massachusetts): Stay granted after third-party lab tests alleged potency discrepancies.
Potter v. PotNetwork (2020, Florida): One of the rare cases that proceeded, though it eventually settled.
In most instances, the courts pressed pause.
What This Means for the Hemp and CBD Industry
Labeling & Certificates of Analysis (COAs): Plaintiffs often base lawsuits on third-party testing. Inaccurate COAs or potency statements create legal risk.
Health & Disease Claims: Unsubstantiated claims (e.g., “CBD cures anxiety”) are a common trigger for litigation.
Litigation Strategy: Defense attorneys frequently invoke primary jurisdiction to argue for dismissal or a stay.
For businesses, this means that regulatory compliance isn’t optional—it’s your first line of defense in court.
Why Expert Witnesses Matter in Hemp Litigation
As an expert witness in cannabis and hemp cases, I help courts, attorneys, and regulators understand:
Analytical testing methods: Whether a lab used proper equipment and controls.
Industry standards: What’s normal practice versus negligent conduct.
Regulatory context: How FDA, USDA, and state laws intersect in labeling disputes.
This technical expertise can make the difference between a dismissed case and a costly judgment.
Closing Thought
Primary jurisdiction has acted as a legal “pause button” on CBD litigation, but it’s not a permanent solution. Once FDA finalizes its hemp and CBD regulations, the courts will move forward—and businesses will need to show that their labels, testing, and marketing practices meet the standard.
Until then, hemp litigation remains a waiting game, with expert witnesses playing a critical role in bridging the gap between science, regulation, and the law.
The information provided in this article is for educational and informational purposes only and does not constitute legal advice. Reading this post does not create an attorney–client relationship. Individuals or businesses facing hemp or CBD-related litigation should consult with a qualified attorney for advice regarding their specific situation.